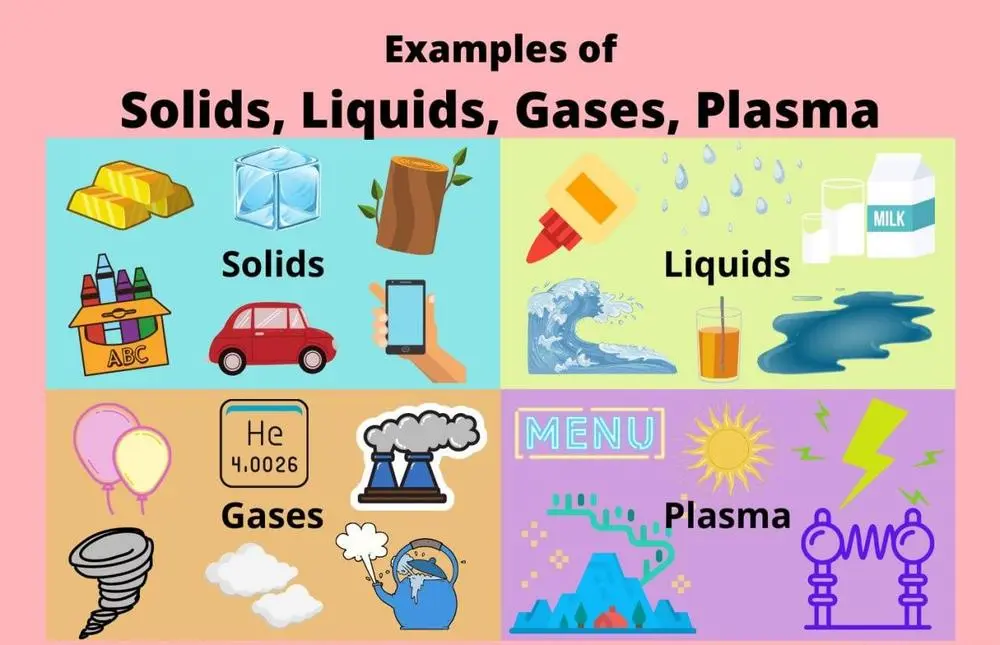
**How are the main states of matter defined?**
📍 **Solid**
is a state of matter with a defined shape and volume. Atoms, ions, and molecules in a solid pack tightly together and may form crystals.
**📍 Liquid**
is a state of matter with a defined volume, but no defined shape, as liquids take the shape of their container. Particles in a liquid have more energy than in a solid, so they are further apart and less organized (more random).
📍 **Gas**
is a state of matter lacking either a defined volume (like a liquid) or defined shape easily expanding or contracting (unlike a liquid). Particles in a gas have more energy and move more randomly than in solids or liquids.
📍 **Plasma**
is a state of matter similar to a gas, except all of the particles carry an electrical charge, exist at very low pressure and are even further apart than in a gas. Plasma can consist of ions, electrons, or protons. Examples of plasma include lightning, the aurora, the Sun, and the inside of a neon sign.
Subscribe- t.me/askmenow